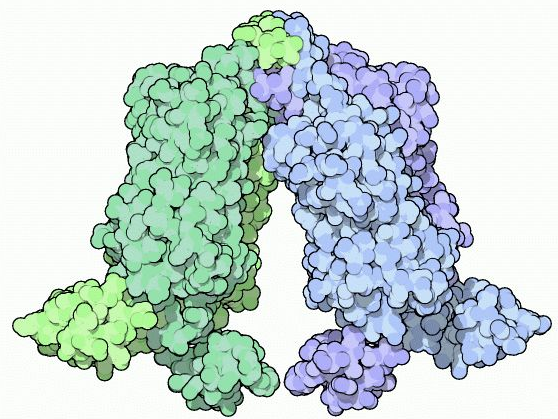
Mitchell Lewis and coworkers obtained the crystal structure for the Lac repressor bound to IPTG or lac DNA in 1996. It shows the structure of one of the four identical Lac repressor subunits. As indicated in the figure, the monomer has four distinct functional units:
1. N-terminal domain or headpiece (residues 1-45) binds lac operator DNA. A helix-turn-helix motif within the head-piece recognizes and binds appropriate operator DNA. Helix-turn-helix motifs, which are often present in proteins that bind to DNA, consist of two short α-helical segments containing between 7 and 9 residues that are separated by a β-turn (a tight turn involving four amino acid residues). One of the two α-helices, designated the recognition helix, is positioned in the major groove of the DNA so that amino acid residues within this helix make specific contacts with bases in the DNA. The other helix, which is designated the stabilization helix, helps to stabilize the complex.
2. The hinge region (residues 46-62), which joins the N-terminal headpiece to the core domain, is disordered in the absence of DNA but folds into an α-helix upon binding to operator DNA. Two hinge helices in a Lac repressor dimer associate through van der Waals interactions and the paired hinge helices bind to the center of the lac operator in the minor groove and bend the DNA.
3. The ligand binding or core domain (residues 62-340) is divided into an N-terminal sub-domain and a C-terminal sub-domain. The two kinds of sub-domain have similar folding patterns, Weak non-covalent interactions between N-terminal sub-domains as well as between C-terminal sub-domains make important contributions to Lac repressor dimer formation. IPTG fits in a pocket between the two sub-domains in each polypeptide.
4. The tetramerization helix (residues 341-357) associates with corresponding regions of the other three subunits of the Lac repressor to form a four-helix bundle that produces a Lac repressor tetramer with a V-shape. Mutations or deletions that alter the tetramerization helix result in the formation of dimers rather than tetramers. The dimers have partial repressor function.
The crystal structures of Lac repressor bound to DNA or IPTG suggest a possible mechanism for the Lac repressor to act as a molecular switch that turns the Lac system off and on. Kathleen Matthews and colleagues have suggested the model to explain how a Lac repressor dimer might work. According to this model, the helix-turn-helix motif in each N-terminal domain makes contact with bases in the major groove of a Lac operator half-site. Furthermore, the hinge helices insert into the minor groove of the central region of the structure, causing the DNA to bend. Binding IPTG to the Lac repressor causes the N-terminal sub-domain of the core region to move with respect to the C-terminal sub-domain of the core region. This movement is similar to screwing on a cap (with both translation and rotation), closing the IPTG-binding site and inducing a conformational change that disrupts the structures of the hinge helices. Disruption of the. Hinge helix structure frees the helix-turn-helix motif to leave the operator DNA.